When vegetable oils self-heat, the first chemical compounds to diminish in concentration are the polyunsaturated fatty acids (PUFAs) such as linoleic and linolenic acids. At high temperatures or under prolonged heating the PUFAs can diminish to the extent they are no longer detectable. The presence of PUFAs in the analysis of fire debris is an essential element in determining whether self-heating is a possibility or not (e.g. ASTM E2881). Once PUFAs have reacted, additional means of characterising the oils or fats present are needed.
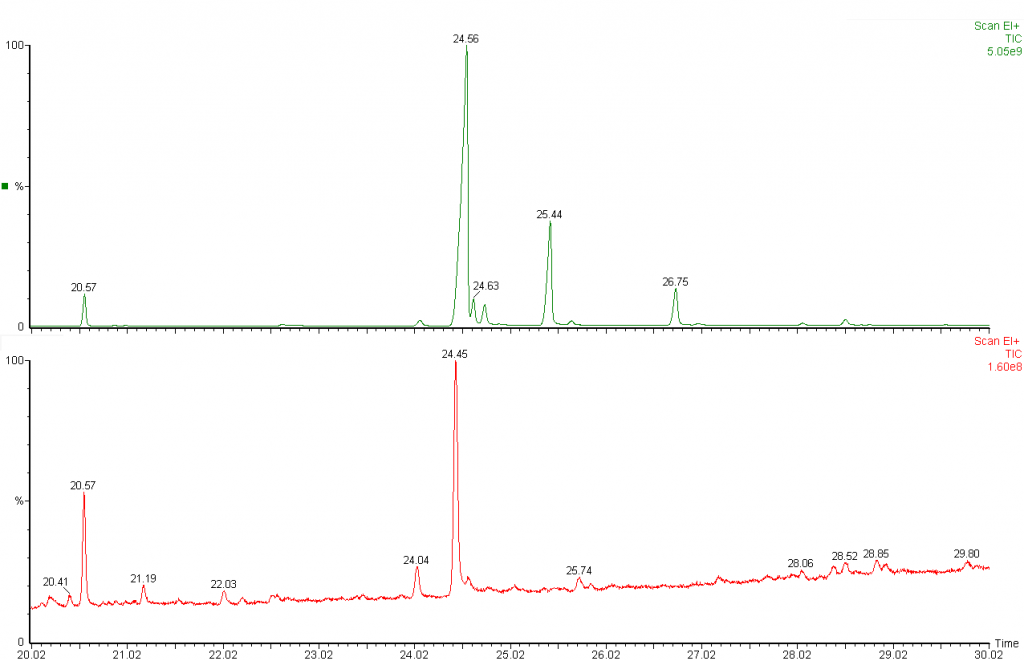
Chromatograms of rapeseed oil prior to heating (green) and after heating (red) showing loss of PUFAs (peaks between 24.45 and 29 min)
SMS Analytical have been identifying some oxidation products of PUFAs in fire debris to help us establish whether any oils and fats that are present are capable of self-heating. We are continuing to improve our analyses and interpretation of results to provide more detailed reports for fire-related problems.
Contact us to discuss ways we can assist in fire debris analyses